Science
We Are All Star Stuff: Do You Know About The Astrobiology Periodic Table?
- How cool is this: a version of the periodic table that tells you each element’s astrophysical origin and its biological use.
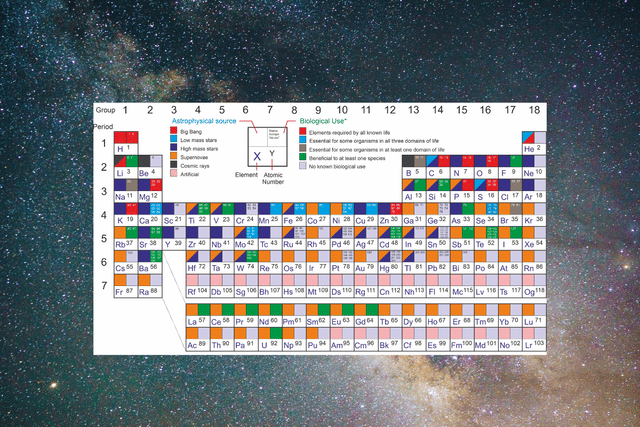
A version of the periodic table useful in astrobiology
The periodic table of elements is a rite of passage for high-school students. Memorising the table not only got you marks on the exam, it helped you earn brownie points among classmates if you were able to rattle off at least some of the names situated lower down in the table (such as the “lanthanides” and “actinides”).
The periodic table, created by Russian chemist Dmitri Mendeleev in 1871 (his initial 1869 table didn’t have periodicity), organises all the known elements by atomic number, which is the number of protons in each atom of the element. It is managed by the International Union of Pure and Applied Chemistry, or IUPAC.
What’s interesting, though, is that this all-familiar table has a very interesting little-known, specialised version. It’s the same periodic table, except it supplies additional information that would come in handy for scientists in a particular area of focus — astrobiology. (Though it would prove useful in astrochemistry and biochemistry as well).
Charles Cockell, Professor of Astrobiology at the University of Edinburgh, had devised this table back in June 2015. That was “v. 1.0” (version one), and it was called the ‘astrobiological’ periodic table.
Now, nearly eight years later, we have version six — v. 6.0 — of the ‘astrobiology’ periodic table. Prof Cockell on 17 February shared this latest version on Twitter.
The updated table is said to incorporate new biological data from a recent paper by Kaleigh A Remick and John D Helmann, called The elements of life: A biocentric tour of the periodic table and published in Advances in Microbial Physiology. The paper provides “a global survey of how chemical elements contribute to life,” thus forming the basis for v. 6.0.
Prof Cockell, author of the undergraduate textbook Astrobiology: Understanding Life in the Universe, had already tweeted last month that he needed to update the astrobiology periodic table because rare earth elements “have known cofactor roles in enzymes” and “astrophysical sources are better understood”.
Astrobiology is defined in Astrobiology: A Very Short Introduction by David C Catling as a branch of science concerned with the study of the origin and evolution of life on Earth and the possible variety of life elsewhere.
The study of life on Earth thus gives us a clue about life “out there.” When we use the very pretty cliché “We are all made of stardust,” the “we” in that line covers all beings in the universe presumably.
Most of the elements (some are created artificially in laboratories) come from various objects, events, or processes that have either already taken place or are occurring regularly in the universe — the big bang, the birth and death of stars (especially supernovae), and cosmic rays, to name a few.
That means we can trace back every element to where it came from and, based on what we know from biological research, its particular function(s) within biology.
Hence, we have the “astrobiology periodic table”.
The astrobiology periodic table thus exists to provide two specific points of information — for every element, its astrophysical source as well as its biological use, colour-coded for clear and easy reference.
For example, Hydrogen (H1), occupying pole position on the periodic table, came from the big bang and is an element required by all known life.
Similarly, Beryllium (Be4), just under hydrogen on the table, comes from cosmic rays and happens to have no known biological use.
Such information is neatly presented for every element, as is the purpose, on the astrobiology periodic table.
It also makes one wonder, as scientist Jethro Hemmann does in a tweet, “if we will discover biological utilization for some of these other elements in the future?”
Only a couple of weeks ago, on 7 February, was Periodic Table Day.
Though it’s a toast to the publishing of English chemist John Newlands’ periodic table of the elements on 7 February 1863, the birth anniversary of Mendeleev, whose periodic table is what we study in chemistry class, falls only a day later, on 8 February (birth year 1834).
Either way, it’s been over 150 years since the invention of the periodic table of the elements. Thus, the arrival of this latest version of the astrobiology periodic table is nicely timed!
Now, I’m off to go spend some more time with it.
Meanwhile, here’s a fun song by American singer-songwriter Tom Lehrer. Made in 1959, the song The Elements delivers the periodic table to the tune of another song, I Am the Very Model of a Modern Major-General, from theatre duo Gilbert and Sullivan's comic opera The Pirates of Penzance.
Support Swarajya's 50 Ground Reports Project & Sponsor A Story
Every general election Swarajya does a 50 ground reports project.
Aimed only at serious readers and those who appreciate the nuances of political undercurrents, the project provides a sense of India's electoral landscape. As you know, these reports are produced after considerable investment of travel, time and effort on the ground.
This time too we've kicked off the project in style and have covered over 30 constituencies already. If you're someone who appreciates such work and have enjoyed our coverage please consider sponsoring a ground report for just Rs 2999 to Rs 19,999 - it goes a long way in helping us produce more quality reportage.
You can also back this project by becoming a subscriber for as little as Rs 999 - so do click on this links and choose a plan that suits you and back us.
Click below to contribute.
Latest