Insta
India's Drug Regulator DCGI Exempts Local Bridging Trials For Foreign Vaccines Approved By WHO And Select Overseas Regulators
Swarajya Staff
Jun 02, 2021, 11:01 AM | Updated 11:01 AM IST
Save & read from anywhere!
Bookmark stories for easy access on any device or the Swarajya app.
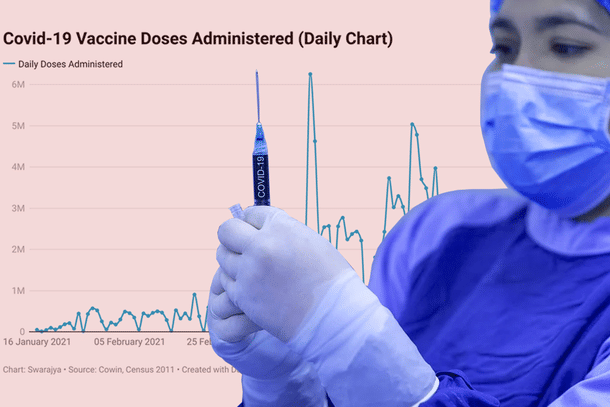
Based on the recommendations by the National Expert Group on Vaccine Administration for COVID-19 (NEGVAC), India's drug regulator Drug Controller General of India (DCGI) has done away with the requirement of India-specific trials
The move is likely to pave way for foreign vaccines like Pfizer and Moderna, to the Indian market and is likely to help accelerate India's Covid-19 vaccination program
Foreign vaccines approved by specific countries and WHO for emergency use will no longer need bridging trials in India, the Drug Controller General of India (DCGI) has ruled.
A DCGI letter says it has waived the requirement for foreign companies to carry out "post-launch bridging trials" and to test the quality and stability of their vaccines in India if they have approvals from specific countries or health bodies.
The exemption will, however, be limited to COVID-19 vaccines approved in India for restricted use in emergency situation which are already approved for restricted use by USFDA, EMA, UK MHRA, PMDA Japan or listed in WHO Emergency Use Listing (EUL) and which are well established vaccine from the stand point that millions of individuals have already been vaccinated with the said vaccines.
The requirement of assessment on the first 100 beneficiaries for 7 days for safety outcomes before the vaccine is rolled out for further immunization programme along with other procedures for filing of applications and timelines for processing of the applications, etc. as laid down in the notice on April 15 will remain.
The government earlier insisted on local clinical trials or bridging studies that involves testing the vaccine in Indian participants to assess the safety and immunogencity in the local population for vaccines developed overseas.
DCGI also said the imported vaccine batches will have to be certified and released by National Control Laboratory of Country of Origin.
The DCGI chief, VG Somani, has said in the letter that the decision was taken "in the light of the huge vaccination requirements in India in the wake of the recent surge of COVID-19 cases and the need for increased availability of imported vaccines".
Pfizer and Moderna were among the companies that had requested the government for waivers like indemnity and for post-approval local trials. The government has yet to take a decision on indemnity or liability from compensation for any severe side effects.





