News Brief
Nasal Vaccines Could Be ‘Game Changers’ For Children In India But Roll Out In 2021 Unlikely: WHO’s Soumya Swaminathan
Bhaswati Guha Majumder
May 25, 2021, 04:58 PM | Updated 04:58 PM IST
Save & read from anywhere!
Bookmark stories for easy access on any device or the Swarajya app.

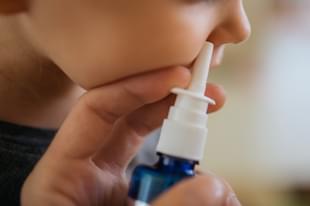
World Health Organisation’s Chief Scientist Dr Soumya Swaminathan said on 23 May that the nasal Covid-19 vaccines which are under development could be a “game-changer” in India.
But the WHO expert noted that the nasal vaccines may not be available in the country this year, while there is a possibility that in the coming months a third wave of the coronavirus pandemic could hit children in India.
She told CNN-News18: “Some of the nasal vaccines that are going to be made in India could be game-changers for children — easy to administer, will give you local immunity in the respiratory tract.”
Earlier Randeep Guleria, who is the director of All India Institute of Medical Sciences, said that nasal vaccines like Bharat Biotech’s BBV154 “will be very easy to be given to children as it is a spray and not a jab and hence compliance is more".
The Hyderabad-based Bharat Biotech, which developed Covaxin, has already begun the process of screening potential clinical trial participants at four centres in India - Nagpur, Patna, Chennai and Hyderabad for their nasal vaccine against Covid-19.
The Indian biotechnology company announced the collaboration with Washington University School of Medicine in September 2020 to develop this single-dose intranasal vaccine.
While the first phase trials were reported to have proven successful, efforts to complete the subsequent second and third phase clinical trials are also going on.
The Pune based Serum Institute of India (SII) has also kickstarted phase one trials of its nasal Covid-19 vaccine in the UK.
SII and New York-based vaccine maker Codagenix collaborated to develop this nasal vaccine, named COVI-VAC.
WHO scientist Swaminathan said she is very hopeful that ultimately India will have the vaccine for children.
“But that’s not going to happen this year and we should open schools when community transmission is down,” she added.
According to her, in such a case, if teachers are vaccinated, “that would be a big step forward”.
Meanwhile, in the United States, the Food and Drug Administration (FDA) has given emergency approval to the Pfizer-BioNTech Covid-19 vaccine for youngsters aged between 12 and 17.
In Singapore, the authorities have also approved Pfizer-BioNTech’s mRNA vaccine for children aged 12 to 15.
But in India, as of 25 May, regulators haven’t approved any vaccines for children.
According to reports, Bharat Biotech is planning to launch the nasal vaccine in the country.
In early May Bharath Biotech’s joint managing director Suchitra Ella said: “Am hopeful that the final stage clinical trials will be completed in the next 3-4 months and within next 6 months we will be able to launch the nasal vaccine in India.”





