Insta
Phase 1 Clinical Trial For Experimental Coronavirus Vaccine Begins In United States’ Seattle
Swarajya Staff
Mar 17, 2020, 10:04 AM | Updated 10:04 AM IST
Save & read from anywhere!
Bookmark stories for easy access on any device or the Swarajya app.
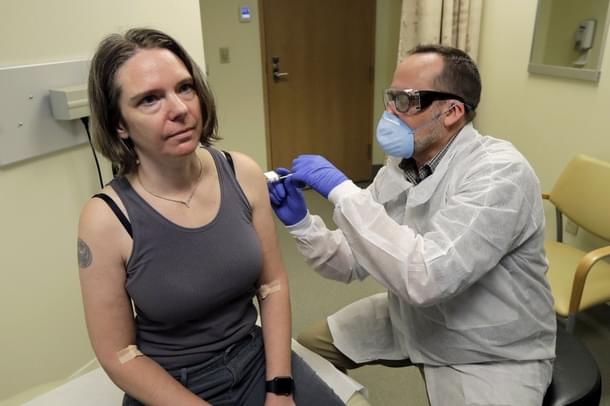
Amid the worldwide surge in the number of patients of the novel Coronavirus, the US Researchers on Monday (16 March) started the clinical trial of an experimental vaccine that is designed to protect against the Covid-19.
The phase 1 clinical trial began at the Kaiser Permanente Washington Health Research Institute in Seattle, funded by National Institute of Healths, according to a US Department of Health and Human Services (HHS) statement.
The first participant, Jennifer Haller, an operations manager at a small tech company, received the injection inside an exam room. Three others were next in line for a test that would ultimately give 45 volunteers two doses, a month apart, reports Los Angeles Times.
The study is evaluating different doses of the experimental vaccine for safety and its ability to induce an immune response in participants. The vaccine was developed by NIAID scientists and their collaborators at the biotechnology company Moderna, Inc. The Coalition for Epidemic Preparedness Innovations (CEPI) supported the clinical manufacturing of the vaccine candidate, the statement added.
“NIH and its partners have launched the first COVID-19 vaccine trial anywhere in the world, in record-breaking time,” said HHS Secretary Alex Azar, adding that the US is using its best minds and all the resources to protect its people.
The Seattle experiment got underway just days after the World Health Organization called the new virus outbreak a pandemic because of its rapid global spread, infecting more than 169,000 people and killing more than 6,500.





